Abstract

Introduction T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL) comprises approximately 15% of ALL cases. Most cases occur in adolescent and young adult males. Most patients with T-ALL/LBL initially respond to chemotherapy. However, relapsed/refractory (R/R) T-ALL has a dismal prognosis due to few treatment options and chemotherapy resistance. Nelarabine, a pro-drug of nucleoside analog (Ara-G) is the only drug approved for R/R T-ALL/LBL based on phase 2 trials. To improve outcomes, nelarabine has been combined with cyclophosphamide and etoposide (NECTAR) in a few small studies. Here, we report our experience with nelarabine monotherapy and nelarabine combination regimens in adult and pediatric patients with R/R T-ALL/LBL.
Methods We retrospectively identified consecutive patients with R/R T-ALL/LBL who were treated with at least one dose of nelarabine between August 2006 and November 2021 at the Boston Children's Hospital, Dana-Farber Cancer Institute/Brigham and Women's Hospital, or Massachusetts General Hospital. Outcome measures include rate of complete remission (CR), measurable residual disease (MRD) by flow cytometry, overall survival (OS), relapse free survival (RFS), rate of alloSCT, and hematologic and nonhematologic adverse events (AEs).
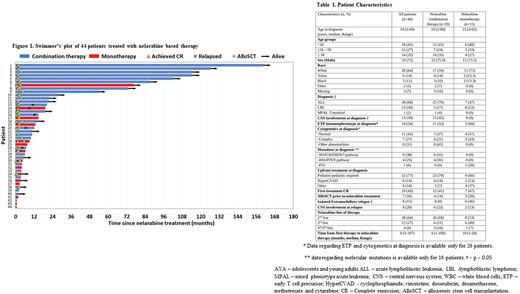
Results We identified 44 patients treated with nelarabine for R/R T-ALL/LBL: 29 (66%) with nelarabine combination (combination group) and 15 (34%) with single agent nelarabine (monotherapy group), without difference between children and adults. Median age was 19 years (range, 2-69), 75% were male and 30% had LBL without marrow involvement (Table 1). The nelarabine dose and schedule was 1500 mg/m2/day days 1, 3, and 5 (n=17) or 650 mg/m2/day for 5 consecutive days (n=27), with or without additional therapy. The most common combination therapy (79%, n=23) was NECTAR (nelarabine, etoposide, and cyclophosphamide).
In terms of toxicity, grade 3-4 anemia and thrombocytopenia were more prevalent among the combination vs monotherapy group (76% vs 20%, p < 0.001 and 66% vs 27%, p=0.014, respectively). Neurotoxicity occurred in 5 (17%) vs 4 (27%) patients in the combination and monotherapy groups (p=0.46), respectively. Neurologic events were either central (n=4) or peripheral (n=5), transient, and grade 3 or less. The only AEs more common in adults were grade 3 infections and grade 3-4 thrombocytopenia (58% vs 11%, p=0.002 and 65% vs 33%, p=0.036, respectively).
For the entire cohort, CR rate was 55% (24/44) and 73% (16/22) of CRs were MRD-negative by flow cytometry. The CR rate was 62% and 40% among the combination and monotherapy groups (p 0.21), respectively. AlloSCT was performed in 21/24 (88%) patients achieving CR. An additional 5 patients pursued alloSCT after additional chemotherapy. Overall, alloSCT was pursued in 19 (66%) and 7 (47%) patients in the nelarabine combination group vs monotherapy group, respectively (p=0.33).
The 2-year RFS among patients who achieved CR was 60.5% (95% CI 36-78%). All relapses following CR occurred in the first year. The 2-year RFS was numerically higher in the combination vs monotherapy group (68.8% [95% CI 41-86%] vs 26.7% [95% CI 1-69%] p= 0.07, respectively).
The median OS for the entire cohort was 12.9 months (95% CI; 7-not reached) and the 2-year OS was 37.6% (95% CI; 22-53%), higher in the combination vs monotherapy group (52.9% [95% CI; 32-70%] vs 8% [95% CI; 1-30%], respectively, p= 0.0026). Among patients who proceeded with Transplant (n=26), the 2-year OS was higher in the combination vs monotherapy group (70.9% [95% CI; 43-87%] vs 17% [95% CI 1-52%], respectively, p-0.002). Median OS did not differ between children and adults, nor by the presence of NOTCH mutation, line of therapy, or ETP vs non-ETP subtype. In a multivariate Cox regression model for OS, both nelarabine combination therapy and alloSCT post nelarabine retained their predictive value (HR 0.41, 95% CI; 0.17-0.96 p=0.04 and HR 0.25, 95% CI; 0.09-0.7 p=0.008).
Conclusion Nelarabine combination chemotherapy is well tolerated in both pediatric and adult patients with R/R T-ALL/LBL, with a higher CR rate and improved OS compared to patients treated with nelarabine monotherapy. As alloSCT is crucial to achieve cure in R/R T-ALL/LBL, nelarabine combination therapy should be considered for treatment as a bridge to transplant.
Disclosures
Place:Novartis: Consultancy, Other: Meal, Research Funding; Abbvie: Consultancy, Research Funding; Servier: Consultancy, Research Funding. Brunner:Takeda: Consultancy, Research Funding; Keros Therapeutics: Consultancy; Novartis: Consultancy, Research Funding; AstraZeneca: Research Funding; Celgene/BMS: Consultancy, Research Funding; GSK: Research Funding; Janssen: Research Funding; Aprea: Research Funding; Agios: Honoraria; Taiho: Consultancy; Acceleron: Honoraria. Silverman:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees. Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. Stone:Jazz: Consultancy; Syndax: Consultancy; OncoNova: Consultancy; Innate: Consultancy; GSK: Consultancy; Astellas: Consultancy; BerGenBio: Consultancy; Apteva: Consultancy; Aprea: Consultancy; Kura Oncology: Consultancy; Epizyme: Consultancy; Foghorn Therapeutics: Consultancy; Gemoab: Consultancy; Novartis: Consultancy; Elevate Bio: Consultancy; Janssen: Consultancy; Arog: Consultancy, Research Funding; Boston Pharmaceuticals: Consultancy; BMS: Consultancy; Syros: Consultancy; Takeda: Consultancy; Syntrix: Consultancy; Actinium: Consultancy; Abbvie: Consultancy, Research Funding. DeAngelo:Forty-Seven: Consultancy; Incyte: Consultancy; Autolus: Consultancy; Agios: Consultancy; Amgen: Consultancy; Glycomimetics: Research Funding; Shire: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Blueprint Medicines Corporation: Consultancy; Abbvie: Research Funding. Luskin:Pfizer: Honoraria; Abbvie: Research Funding; Novartis: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal